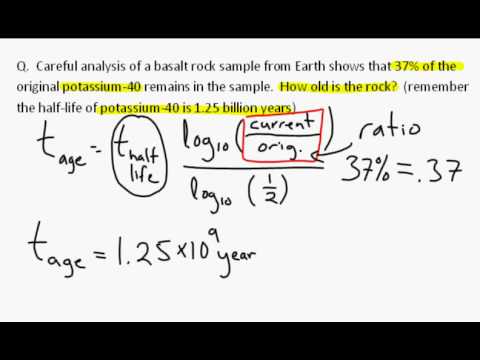
Calculation of radiocarbon dates If the benzene sample contains carbon It should also incorporate errors on every measurement taken as part of the dating. Much of the information presented in this section is based upon the Stuiver and Polach () paper "Discussion: Reporting of C14 data". A copy of this paper may be found in the Radiocarbon Home Page. The radiocarbon age of a sample is obtained by measurement of the residual radioactivity. The radiocarbon age of a sample is obtained by measurement of the You can get an idea of the relationship between C14 and age at the Carbon Dating calculator.
Carbon dating calculation sample -
For older fossils, an isotope with a longer half-life should be used. In , the British Museum radiocarbon laboratory ran weekly measurements on the same sample for six months. The key questions then are: A 10 gram sample of UNow that has changed, and some important discoveries are being made. Using this measurement also corrects for any mass-dependent fractionation within the AMS system. This constant ratio is maintained until the death of an organism, when 14 C stops being replenished. In addition to loss through decay of radiocarbon, 14 C is also affected by natural isotopic fractionation.
Carbon dating calculation sample -
In , the British Museum radiocarbon laboratory ran weekly measurements on the same sample for six months. The different elements of the carbon exchange reservoir vary in how much carbon they store, and in how long it takes for the 14 C generated by cosmic rays to fully mix with them. In addition to permitting more accurate dating within archaeological sites than previous methods, it allows comparison of dates of events across great distances. A Guide to Radiocarbon Units and Calculations , p. The northern and southern hemispheres have atmospheric circulation systems that are sufficiently independent of each other that there is a noticeable time lag in mixing between the two.

Carbon Dating Calculation Sample. Calculation of radiocarbon dates - Wikipedia
For consistency with these early papers, and to avoid the risk of a double correction for the incorrect half-life, radiocarbon ages are still calculated using the incorrect half-life value. The fraction modern is then converted to an age in "radiocarbon years", meaning that the calculation uses Libby's half-life of 5, years, not the more accurate modern value of 5, carbon dating calculation sample, and that no calibration has been done: Thus, ages are limited by the age of the process blanks more on that below and by the statistical uncertainty of the carbon dating calculation sample C measurement. The standard used for modern carbon is wood, with a baseline date of The equation for the radioactive decay of 14 C is:


-
-
-